
Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.
Insulin pumps are used to precisely control the amount of insulin injected in diabetic patients. Patients with more severe conditions may need multiple injections of insulin per day to maintain a reasonable blood sugar level. Insulin pumps can improve the quality of life of patients, and reduce the risk of complications due to long-term illness by rationally controlling blood sugar. The insulin pump firmware design allows for adjustment of the syringe injection rate and adjusts the amount of insulin injected based on the patient's eating, sleep and exercise conditions. The insulin is placed in a user-replaceable needle and the needle is placed in the pump body, thus forming a special syringe with a piston that can be slowly depressed under the control of the insulin pump. The needle outlet is connected to a hose and insulin is injected through the hose into the patient's skin (usually in the abdomen).
The blood glucose meter provides continuous monitoring of diabetic patients by providing real-time blood glucose indicators through a subcutaneous sensor. Each sensor can be used for several days at a time, which eliminates the need for the patient to repeatedly collect blood samples. The future development trend is to further improve the blood glucose detection, response mechanism and the synergy of the entire loop of the automatic adjustment of insulin dosage.
FDA regulation of medical equipmentThe insulin pump is a portable medical device. In the United States, the design and production of insulin pumps are regulated by the US Food and Drug Administration (FDA). This means that its design architecture must meet the requirements of the relevant documents, and their performance must meet the strict regulations of the PDA and the requirements of development testing, production testing, on-site maintenance (Figure 1).
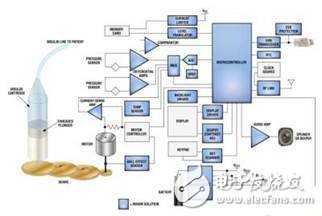
Figure 1: Block diagram of the insulin pump.
The device must also have self-test and fault indication capabilities, which means additional support circuitry and components are required to perform the self-test feature.
Considering the time and cost of obtaining FDA approval, insulin pump manufacturers need to choose a customer-oriented chip supplier that can strictly control the shutdown policy to ensure that system vendors are maintained for several years. Semiconductor manufacturers must
Recognizing the serious damage to device manufacturers caused by discontinued devices, we are constantly relocating some old products to new product lines, establishing wafer inventory, providing last-time procurement opportunities or developing new upgrade replacement products. Maxim rarely discontinues a device when the customer still needs it.
PortabilityInsulin pumps are portable devices that must be small and lightweight (Figure 2). A typical insulin pump measures about 2 & TImes; 3 & TImes; 0.75 inches and weighs only 2 ounces or 4 ounces. This miniaturization requirement requires designers to prioritize size and power consumption when selecting components.
To save space, system designers need highly integrated, ultra-small packaged devices. For example, chip scale packages (Maxim's UCSP package) and wafer level package (WLP). To use batteries that are as small as possible, designers must minimize power consumption and increase efficiency. If possible, put any unused circuitry in shutdown mode.

Figure 2: A typical battery-powered insulin pump system (MiniMed Paradigm 522/722 from Medtronic).
The core component of the system is a highly integrated low standby power microcontroller. For example: MAXQ2010 or a similar MCU. The MAXQ2010 is a 16-bit MCU that operates at 1MHz, consuming only 1mA at 2.7V, and sinking to 370nA in standby mode. This low power consumption can effectively extend the battery life of the insulin pump. Similar to most MCUs, the MAXQ2010 integrates USART, timer, 64-byte flash, 2-byte RAM, and general-purpose I/O functions with a 12-bit successive approximation ADC with a sample rate of 312.5ksps and a built-in reference. Resources such as LCD controllers. The device also features a fast wake-up feature that quickly recovers from sleep and shutdown modes to normal operation, allowing the insulin pump to respond quickly to patient operational needs.
Insulin pump subsystem1. Pump and inspection program
Insulin is measured in "units" and is divided into 100 units per cc (or mL), assuming a standard concentration of U-100. In this metering mode, one unit is equivalent to 10 μL. The injection rate is 1 unit/hour, and each injection is 3 to 10 minutes. The dose of one piece of insulin is several units. Typically, the needle can be filled with 200 to 300 units of insulin.
Taking into account the extremely slow flow rate, the motor drives the gear driven pump to drive the piston of the needle tube to move very slowly. Usually only the angle of the motor needs to be roughly measured. Most insulin pump manufacturers use optical encoders and DC motors, as well as stepper motors. In order to reduce the size of the system, you can also choose to use MEMS pump or pressure pump, thus eliminating motor control.
Use a pressure sensor to detect the seal of the system and ensure proper operation. Based on silicon strain gauges, the output signal amplitude of these sensors is on the order of millivolts, while the output signal range of the bond line strain gauge is on the order of microvolts. The stress meter uses a typical bridge structure to generate a differential signal based on the common-mode voltage. The common-mode voltage is typically half the supply voltage.
The design can use an analog-to-digital converter (ADC) with a differential input programmable gain amplifier (PGA), or a microcontroller with an internal ADC and an external differential amplifier or instrumentation amplifier (for signal conditioning). Pressure measurement does not need to be very high
Accuracy, because the pressure reading is only used to indicate whether the work is normal and not used for injectable dose measurement.
2. Power supply subsystem
Insulin pumps typically use a step-up regulator that boosts the low-voltage (1.5V, nominal) input of a single alkaline battery to 2V or higher. To take full advantage of battery energy, the boost converter should be able to operate at the lowest possible input voltage. The boost converters from Maxim and other power supply manufacturers are capable of operating at voltages as low as 0.6V and start-up voltages as low as 0.7V, effectively increasing battery life.
The MAX1947 step-up DC-DC converter is ideal for this type of application with an input voltage range of 0.7V to 3.6V. The 2MHz switching frequency and current control mode greatly reduces external component size, enabling conversion efficiency greater than 94% and faster response times. The device integrates all of the switching converter circuits (power switches, synchronous rectification, reverse galvanic isolators) to further reduce the size of the solution. A true Shutdown circuit completely disconnects the battery from the load in the off state, helping to further extend battery life.
If the device requires a strictly stable supply voltage, the boosted power supply may need to be further regulated in the design. In such low voltage applications, linear regulators provide higher efficiency due to the absence of switching losses (intrinsic losses in switching power supplies).
In addition, low dropout linear regulators (LDOs) are able to achieve smaller solution sizes, which is especially important for insulin pumps. The efficiency of LDO is very close to the VOUT/VIN ratio. Higher efficiency can be obtained when the difference between VIN and output voltage is slightly higher than the LDO differential.
If the motor requires a regulated source to supply power, a switch mode converter can be selected. To reduce the size and weight, you can choose the converter with the highest switching frequency. For multi-supply systems, a Power Management IC (PMIC) can be selected.
E-mail naar dit bedrijf
January 03, 2024
January 03, 2024

Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.

Fill in more information so that we can get in touch with you faster
Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.